
Safety
Reading time: 5 min
NERLYNX® has a well-understood and predictable safety and tolerability profile2,3,5
Adverse reactions reported in ≥6% of patients in the ExteNET study5
| System Organ Class | Adverse Reaction | All Grades (%) | ≥ Grade 3 (%)† |
|---|---|---|---|
| General Disorders | Fatigue | 27.3 | - |
| Metabolic and Nutritional Disorders | Decreased appetite | 13.7 | - |
| Hepatobiliary Disorders | ALT increased | 8.5 | 1.3 |
| AST increased | 7.4 | 0.7 | |
| Gastrointestinal Disorders |
Diarrhoea |
93.6 |
37.1 |
| Nausea | 42.5 | - | |
| Abdominal pain* | 35.9 | - | |
| Vomiting | 26.8 | 3.5 | |
| Stomatitis | 11.2 | ||
| Skin and Subcutaneous Tissue Disorders | Rash | 15.4 | 0.4 |
| Nail disorders | 7.8 | 0.2 | |
| Musculoskeletal and Connective Tissue Disorders | Muscle spasms | 10.0 | - |
The most frequent adverse event experienced by patients is diarrhoea:
- In the ExteNET study, primary prophylaxis for diarrhoea was not protocol-specified.1
Other than diarrhoea, there is a low incidence of severe adverse events.1
No evidence of cardiac or pulmonary toxicity, nor increased risk for secondary malignancy is observed.1
WARNING: Patients may also experience side effects related to associated treatment, such as hormone therapy.
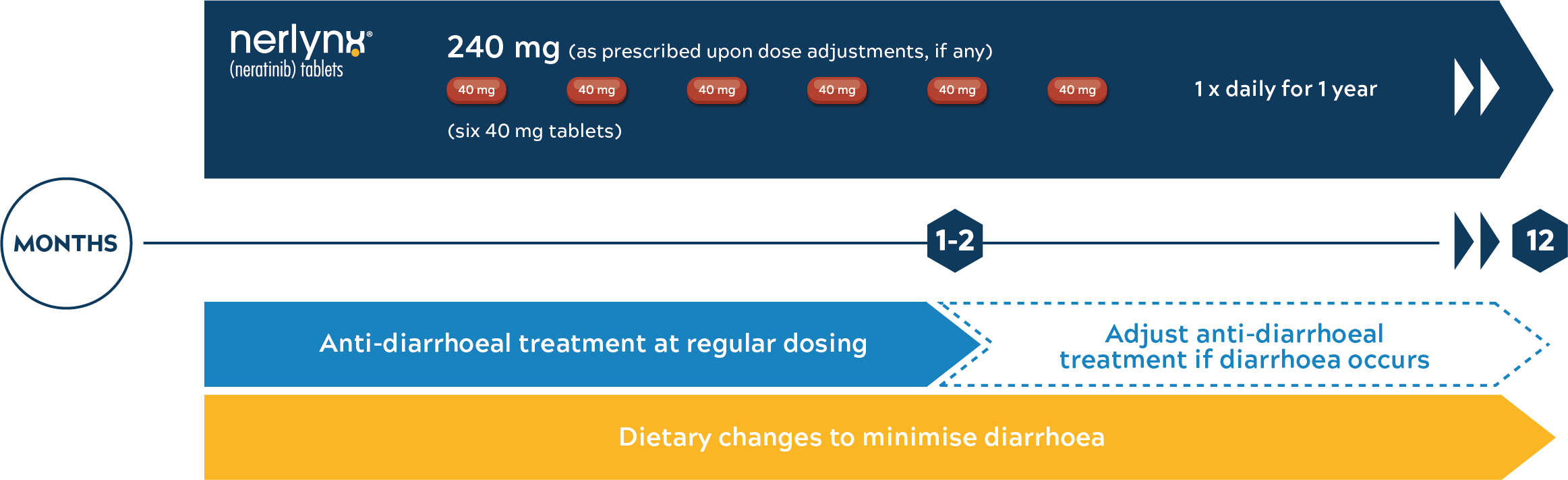
NERLYNX® associated diarrhoea is the most common adverse reaction, it must be monitored and managed
It is recommended to take anti-diarrhoeal prophylaxis with the very first dose of NERLYNX®.5
Dietary recommendations24,25
Diet and lifestyle changes play an important role in the management of NERLYNX® associated diarrhoea. It is important to have a proactive discussion about diet modifications.
Things to do:
Drink more clear liquids
Try to drink ~2L of clear fluids per day.
These include water, sports drinks, broth, weak decaffeinated tea,
caffeine-free soft drinks, clear juices, and gelatin.

Eat small, frequent meals

Choose foods that are easy to digest (low-residue diet).
These include bananas, rice, applesauce and toast.
Things to avoid:

Medicines such as laxatives or stool softeners

Caffeine, alcohol, dairy, fat, fibre, orange juice, grapefruit juice, pomegranate juice, prune juice, and spicy foods
Dose adjustments according to the severity of diarrhoea and based on patient individual safety and tolerability5
The overall management of diarrhoea is based upon its grade as measured by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE).27


