
Possible clinical scenarios
NERLYNX® can be used as extended adjuvant therapy for selected patients*37
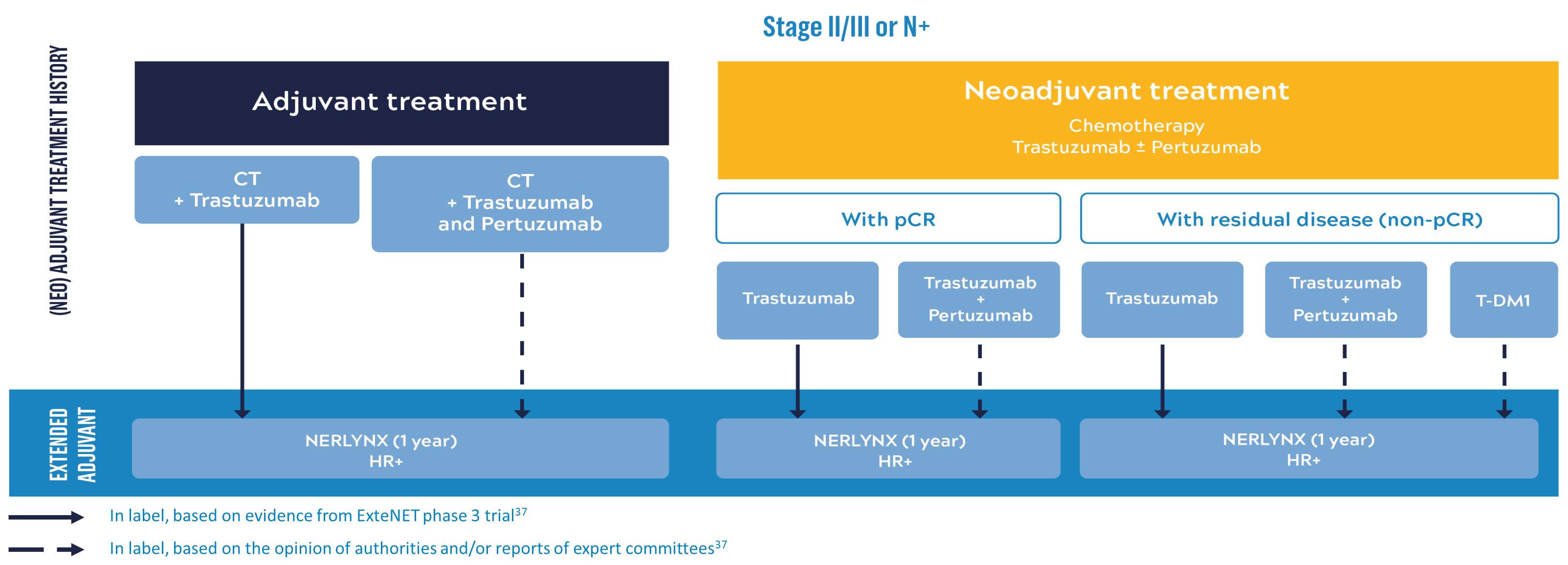
Summary of the possible clinical scenarios taking into account therapeutic advances and acknowledging the different levels of evidence for NERLYNX® use.37

